When referring to the compilation of Henry's Law Constants, please cite
this publication:
R. Sander: Compilation of Henry's law constants (version 5.0.0) for
water as solvent, Atmos. Chem. Phys., 23, 10901-12440 (2023),
doi:10.5194/acp-23-10901-2023
The publication from 2023 replaces that from 2015,
which is now obsolete. Please do not cite the old paper anymore.
|
| FORMULA: | HCN |
|
TRIVIAL NAME:
|
hydrocyanic acid
|
|
CAS RN: | 74-90-8 |
STRUCTURE
(FROM
NIST):
|
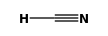
|
|
InChIKey: | LELOWRISYMNNSU-UHFFFAOYSA-N |
|
|
References |
Type |
Notes |
| [mol/(m3Pa)] |
[K] |
|
|
|
| 8.9×10−2 |
8200 |
Burkholder et al. (2019) |
L |
|
| 8.9×10−2 |
8200 |
Burkholder et al. (2015) |
L |
|
| 1.7×10−1 |
4400 |
Yoo et al. (1986) |
L |
1)
|
| 1.1×10−1 |
5000 |
Edwards et al. (1978) |
L |
1)
|
| 8.9×10−2 |
8200 |
Ma et al. (2010a) |
M |
|
| 7.5×10−2 |
|
Riveros et al. (1998) |
M |
12)
|
| 1.2×10−1 |
|
Fredenhagen and Wellmann (1932b) |
M |
|
| 9.2×10−2 |
|
Hine and Weimar (1965) |
R |
|
| 9.9×10−2 |
4200 |
Edwards et al. (1975) |
T |
1)
|
| 7.6×10−2 |
2900 |
Kotlik and Lebedeva (1974) |
X |
566)
|
| 7.4×10−2 |
|
Gaffney and Senum (1984) |
X |
391)
493)
|
| 5.0×10−2 |
|
Keshavarz et al. (2022) |
Q |
|
| 4.2×10−2 |
|
Duchowicz et al. (2020) |
Q |
185)
|
| 3.9×10−2 |
|
Hilal et al. (2008) |
Q |
|
| 1.6×10−1 |
|
Modarresi et al. (2007) |
Q |
68)
|
| 4.7 |
|
Katritzky et al. (1998) |
Q |
|
| 7.4×10−2 |
|
Duchowicz et al. (2020) |
? |
21)
186)
|
| 1.1×10−1 |
|
Yaws (1999) |
? |
21)
|
Data
The first column contains Henry's law solubility constant
at the reference temperature of 298.15 K.
The second column contains the temperature dependence
, also at the
reference temperature.
References
-
Burkholder, J. B., Sander, S. P., Abbatt, J., Barker, J. R., Huie, R. E., Kolb, C. E., Kurylo, M. J., Orkin, V. L., Wilmouth, D. M., & Wine, P. H.: Chemical Kinetics and Photochemical Data for Use in Atmospheric Studies, Evaluation No. 18, JPL Publication 15-10, Jet Propulsion Laboratory, Pasadena, URL https://jpldataeval.jpl.nasa.gov (2015).
-
Burkholder, J. B., Sander, S. P., Abbatt, J., Barker, J. R., Cappa, C., Crounse, J. D., Dibble, T. S., Huie, R. E., Kolb, C. E., Kurylo, M. J., Orkin, V. L., Percival, C. J., Wilmouth, D. M., & Wine, P. H.: Chemical Kinetics and Photochemical Data for Use in Atmospheric Studies, Evaluation No. 19, JPL Publication 19-5, Jet Propulsion Laboratory, Pasadena, URL https://jpldataeval.jpl.nasa.gov (2019).
-
Duchowicz, P. R., Aranda, J. F., Bacelo, D. E., & Fioressi, S. E.: QSPR study of the Henry’s law constant for heterogeneous compounds, Chem. Eng. Res. Des., 154, 115–121, doi:10.1016/J.CHERD.2019.12.009 (2020).
-
Edwards, T. J., Newman, J., & Prausnitz, J. M.: Thermodynamics of aqueous solutions containing volatile weak electrolytes, AIChE J., 21, 248–259, doi:10.1002/AIC.690210205 (1975).
-
Edwards, T. J., Maurer, G., Newman, J., & Prausnitz, J. M.: Vapor-liquid equilibria in multicomponent aqueous solutions of volatile weak electrolytes, AIChE J., 24, 966–976, doi:10.1002/AIC.690240605 (1978).
-
Fredenhagen, K. & Wellmann, M.: Verteilungszahlen des Cyanwasserstoffs und des Wassers über dem Zweistoffsystem [H2O-HCN] bei 18∘C, Z. Phys. Chem., 162A, 467–470, doi:10.1515/ZPCH-1932-16236 (1932b).
-
Gaffney, J. S. & Senum, G. I.: Peroxides, peracids, aldehydes, and PANs and their links to natural and anthropogenic organic sources, in: Gas-Liquid Chemistry of Natural Waters, edited by Newman, L., pp. 5–1–5–7, NTIS TIC-4500, UC-11, BNL 51757 Brookhaven National Laboratory (1984).
-
Hilal, S. H., Ayyampalayam, S. N., & Carreira, L. A.: Air-liquid partition coefficient for a diverse set of organic compounds: Henry’s law constant in water and hexadecane, Environ. Sci. Technol., 42, 9231–9236, doi:10.1021/ES8005783 (2008).
-
Hine, J. & Weimar, Jr., R. D.: Carbon basicity, J. Am. Chem. Soc., 87, 3387–3396, doi:10.1021/JA01093A018 (1965).
-
Katritzky, A. R., Wang, Y., Sild, S., Tamm, T., & Karelson, M.: QSPR studies on vapor pressure, aqueous solubility, and the prediction of water-air partition coefficients, J. Chem. Inf. Comput. Sci., 38, 720–725, doi:10.1021/CI980022T (1998).
-
Keshavarz, M. H., Rezaei, M., & Hosseini, S. H.: A simple approach for prediction of Henry’s law constant of pesticides, solvents, aromatic hydrocarbons, and persistent pollutants without using complex computer codes and descriptors, Process Saf. Environ. Prot., 162, 867–877, doi:10.1016/J.PSEP.2022.04.045 (2022).
-
Kotlik, S. B. & Lebedeva, G. N.: Equilibrium pressures of HCN and NH3 over aqueous solutions, Zh. Prikl. Khim., 47, 444–446 (1974).
-
Ma, J., Dasgupta, P. K., Blackledge, W., & Boss, G. R.: Temperature dependence of Henry’s law constant for hydrogen cyanide. Generation of trace standard gaseous hydrogen cyanide, Environ. Sci. Technol., 44, 3028–3034, doi:10.1021/ES1001192 (2010a).
-
Modarresi, H., Modarress, H., & Dearden, J. C.: QSPR model of Henry’s law constant for a diverse set of organic chemicals based on genetic algorithm-radial basis function network approach, Chemosphere, 66, 2067–2076, doi:10.1016/J.CHEMOSPHERE.2006.09.049 (2007).
-
Riveros, P. A., Koren, D., McNamara, V. M., & Binvignat, J.: Cyanide recovery from a gold mill barren solution containing high levels of copper, CIM Bull., 91, 73–81 (1998).
-
Yaws, C. L.: Chemical Properties Handbook, McGraw-Hill, Inc., ISBN 0070734011 (1999).
-
Yoo, K.-P., Lee, S. Y., & Lee, W. H.: Ionization and Henry’s law constants for volatile, weak electrolyte water pollutants, Korean J. Chem. Eng., 3, 67–72, doi:10.1007/BF02697525 (1986).
Type
Table entries are sorted according to reliability of the data, listing
the most reliable type first: L) literature review, M) measured, V)
VP/AS = vapor pressure/aqueous solubility, R) recalculation, T)
thermodynamical calculation, X) original paper not available, C)
citation, Q) QSPR, E) estimate, ?) unknown, W) wrong. See Section 3.1
of Sander (2023) for further details.
Notes
| 1) |
A detailed temperature dependence with more than one parameter is available in the original publication. Here, only the temperature dependence at 298.15 K according to the van 't Hoff equation is presented. |
| 12) |
Value at T = 293 K. |
| 21) |
Several references are given in the list of Henry's law constants but not assigned to specific species. |
| 68) |
Modarresi et al. (2007) use different descriptors for their calculations. They conclude that a genetic algorithm/radial basis function network (GA/RBFN) is the best QSPR model. Only these results are shown here. |
| 185) |
Value from the validation set for checking whether the model is satisfactory for compounds that are absent from the training set. |
| 186) |
Experimental value, extracted from HENRYWIN. |
| 391) |
Value given here as quoted by Gaffney et al. (1987). |
| 493) |
Value at pH = 4. |
| 566) |
Value given here as quoted by Ma et al. (2010a). |
The numbers of the notes are the same as
in Sander (2023). References cited in the notes can be
found here.
|
* * *
Search Henry's Law Database
* * *
Convert Henry's Law Constants
* * *
|

