When referring to the compilation of Henry's Law Constants, please cite
this publication:
R. Sander: Compilation of Henry's law constants (version 5.0.0) for
water as solvent, Atmos. Chem. Phys., 23, 10901-12440 (2023),
doi:10.5194/acp-23-10901-2023
The publication from 2023 replaces that from 2015,
which is now obsolete. Please do not cite the old paper anymore.
|
| FORMULA: | C12H4Br6O |
|
TRIVIAL NAME:
|
PBDE-153
|
|
CAS RN: | 68631-49-2 |
STRUCTURE
(FROM
NIST):
|
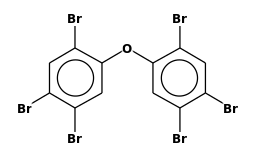
|
|
InChIKey: | RZXIRSKYBISPGF-UHFFFAOYSA-N |
|
|
References |
Type |
Notes |
| [mol/(m3Pa)] |
[K] |
|
|
|
| 3.7 |
7200 |
Long et al. (2017) |
M |
288)
|
| 3.5 |
7800 |
Cetin and Odabasi (2005) |
M |
|
| 6.1 |
|
Kuramochi et al. (2014) |
V |
|
| 1.5×101 |
|
Tittlemier et al. (2002) |
V |
|
| 2.9 |
|
Wania and Dugani (2003) |
R |
|
| 3.9 |
7400 |
Long et al. (2017) |
Q |
288)
|
| 8.4×10−1 |
|
Hilal et al. (2008) |
Q |
|
Data
The first column contains Henry's law solubility constant
at the reference temperature of 298.15 K.
The second column contains the temperature dependence
, also at the
reference temperature.
References
-
Cetin, B. & Odabasi, M.: Measurement of Henry’s law constants of seven polybrominated diphenyl ether (PBDE) congeners as a function of temperature, Atmos. Environ., 39, 5273–5280, doi:10.1016/J.ATMOSENV.2005.05.029 (2005).
-
Hilal, S. H., Ayyampalayam, S. N., & Carreira, L. A.: Air-liquid partition coefficient for a diverse set of organic compounds: Henry’s law constant in water and hexadecane, Environ. Sci. Technol., 42, 9231–9236, doi:10.1021/ES8005783 (2008).
-
Kuramochi, H., Takigami, H., Scheringer, M., & Sakai, S.: Measurement of vapor pressures of selected PBDEs, hexabromobenzene, and 1,2-bis(2,4,6-tribromophenoxy)ethane at elevated temperatures, J. Chem. Eng. Data, 59, 8–15, doi:10.1021/JE400520E (2014).
-
Long, J., Youli, Q., & Yu, L.: Effect analysis of quantum chemical descriptors and substituent characteristics on Henry’s law constants of polybrominated diphenyl ethers at different temperatures, Ecotoxicol. Environ. Saf., 145, 176–183, doi:10.1016/J.ECOENV.2017.07.024 (2017).
-
Tittlemier, S. A., Halldorson, T., Stern, G. A., & Tomy, G. T.: Vapor pressures, aqueous solubilities, and Henry’s law constants of some brominated flame retardants, Environ. Toxicol. Chem., 21, 1804–1810, doi:10.1002/ETC.5620210907 (2002).
-
Wania, F. & Dugani, C. B.: Assessing the long-range transport potential of polybrominated diphenyl ethers: A comparison of four multimedia models, Environ. Toxicol. Chem., 22, 1252–1261, doi:10.1002/ETC.5620220610 (2003).
Type
Table entries are sorted according to reliability of the data, listing
the most reliable type first: L) literature review, M) measured, V)
VP/AS = vapor pressure/aqueous solubility, R) recalculation, T)
thermodynamical calculation, X) original paper not available, C)
citation, Q) QSPR, E) estimate, ?) unknown, W) wrong. See Section 3.1
of Sander (2023) for further details.
Notes
| 288) |
Data taken from the supplement. |
The numbers of the notes are the same as
in Sander (2023). References cited in the notes can be
found here.
|
* * *
Search Henry's Law Database
* * *
Convert Henry's Law Constants
* * *
|

