When referring to the compilation of Henry's Law Constants, please cite
this publication:
R. Sander: Compilation of Henry's law constants (version 5.0.0) for
water as solvent, Atmos. Chem. Phys., 23, 10901-12440 (2023),
doi:10.5194/acp-23-10901-2023
The publication from 2023 replaces that from 2015,
which is now obsolete. Please do not cite the old paper anymore.
|
| FORMULA: | C10H16 |
|
TRIVIAL NAME:
|
R-(+)-limonene; D-limonene
|
|
CAS RN: | 5989-27-5 |
STRUCTURE
(FROM
NIST):
|
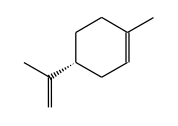
|
|
InChIKey: | XMGQYMWWDOXHJM-SNVBAGLBSA-N |
|
|
References |
Type |
Notes |
| [mol/(m3Pa)] |
[K] |
|
|
|
| 3.8×10−4 |
|
Schuhfried et al. (2015) |
M |
|
| 2.6×10−4 |
|
Helburn et al. (2008) |
M |
|
| 3.5×10−4 |
4500 |
Copolovici and Niinemets (2005) |
M |
|
| 3.8×10−4 |
|
Duchowicz et al. (2020) |
V |
187)
|
| 3.9×10−4 |
|
HSDB (2015) |
V |
|
| 3.8×10−4 |
|
Mackay et al. (2006a) |
V |
|
| 3.8×10−4 |
|
Yaws (2003) |
X |
259)
|
| 3.8×10−4 |
|
Yaws (2003) |
X |
238)
|
| 7.3×10−4 |
|
Dupeux et al. (2022) |
Q |
260)
|
| 6.4×10−4 |
|
Duchowicz et al. (2020) |
Q |
|
| 1.2×10−4 |
|
Gharagheizi et al. (2012) |
Q |
|
| 3.8×10−4 |
|
Gharagheizi et al. (2010) |
Q |
247)
|
| 1.9×10−4 |
|
Modarresi et al. (2007) |
Q |
68)
|
| 3.8×10−4 |
|
Yaws (1999) |
? |
21)
|
Data
The first column contains Henry's law solubility constant
at the reference temperature of 298.15 K.
The second column contains the temperature dependence
, also at the
reference temperature.
References
-
Copolovici, L. O. & Niinemets, U.: Temperature dependencies of Henry’s law constants and octanol/water partition coefficients for key plant volatile monoterpenoids, Chemosphere, 61, 1390–1400, doi:10.1016/J.CHEMOSPHERE.2005.05.003 (2005).
-
Duchowicz, P. R., Aranda, J. F., Bacelo, D. E., & Fioressi, S. E.: QSPR study of the Henry’s law constant for heterogeneous compounds, Chem. Eng. Res. Des., 154, 115–121, doi:10.1016/J.CHERD.2019.12.009 (2020).
-
Dupeux, T., Gaudin, T., Marteau-Roussy, C., Aubry, J.-M., & Nardello-Rataj, V.: COSMO-RS as an effective tool for predicting the physicochemical properties of fragrance raw materials, Flavour Fragrance J., 37, 106–120, doi:10.1002/FFJ.3690 (2022).
-
Gharagheizi, F., Abbasi, R., & Tirandazi, B.: Prediction of Henry’s law constant of organic compounds in water from a new group-contribution-based model, Ind. Eng. Chem. Res., 49, 10 149–10 152, doi:10.1021/IE101532E (2010).
-
Gharagheizi, F., Eslamimanesh, A., Mohammadi, A. H., & Richon, D.: Empirical method for estimation of Henry’s law constant of non-electrolyte organic compounds in water, J. Chem. Thermodyn., 47, 295–299, doi:10.1016/J.JCT.2011.11.015 (2012).
-
Helburn, R., Albritton, J., Howe, G., Michael, L., & Franke, D.: Henry’s law constants for fragrance and organic solvent compounds in aqueous industrial surfactants, J. Chem. Eng. Data, 53, 1071–1079, doi:10.1021/JE700418A (2008).
-
HSDB: Hazardous Substances Data Bank, TOXicology data NETwork (TOXNET), National Library of Medicine (US), URL https://www.nlm.nih.gov/toxnet/Accessing_HSDB_Content_from_PubChem.html (2015).
-
Mackay, D., Shiu, W. Y., Ma, K. C., & Lee, S. C.: Handbook of Physical-Chemical Properties and Environmental Fate for Organic Chemicals, vol. I of Introduction and Hydrocarbons, CRC/Taylor & Francis Group, doi:10.1201/9781420044393 (2006a).
-
Modarresi, H., Modarress, H., & Dearden, J. C.: QSPR model of Henry’s law constant for a diverse set of organic chemicals based on genetic algorithm-radial basis function network approach, Chemosphere, 66, 2067–2076, doi:10.1016/J.CHEMOSPHERE.2006.09.049 (2007).
-
Schuhfried, E., Aprea, E., Märk, T. D., & Biasioli, F.: Refined measurements of Henry’s law constant of terpenes with inert gas stripping coupled with PTR-MS, Water Air Soil Pollut., 226, 120, doi:10.1007/S11270-015-2337-2 (2015).
-
Yaws, C. L.: Chemical Properties Handbook, McGraw-Hill, Inc., ISBN 0070734011 (1999).
-
Yaws, C. L.: Yaws’ Handbook of Thermodynamic and Physical Properties of Chemical Compounds, Knovel: Norwich, NY, USA, ISBN 1591244447 (2003).
Type
Table entries are sorted according to reliability of the data, listing
the most reliable type first: L) literature review, M) measured, V)
VP/AS = vapor pressure/aqueous solubility, R) recalculation, T)
thermodynamical calculation, X) original paper not available, C)
citation, Q) QSPR, E) estimate, ?) unknown, W) wrong. See Section 3.1
of Sander (2023) for further details.
Notes
| 21) |
Several references are given in the list of Henry's law constants but not assigned to specific species. |
| 68) |
Modarresi et al. (2007) use different descriptors for their calculations. They conclude that a genetic algorithm/radial basis function network (GA/RBFN) is the best QSPR model. Only these results are shown here. |
| 187) |
Estimation based on the quotient between vapor pressure and water solubility, extracted from HENRYWIN. |
| 238) |
Value given here as quoted by Gharagheizi et al. (2010). |
| 247) |
Calculated using a combination of a group contribution method and neural networks. |
| 259) |
Value given here as quoted by Dupeux et al. (2022). |
| 260) |
Calculated using the COSMO-RS method. |
The numbers of the notes are the same as
in Sander (2023). References cited in the notes can be
found here.
|
* * *
Search Henry's Law Database
* * *
Convert Henry's Law Constants
* * *
|

