When referring to the compilation of Henry's Law Constants, please cite
this publication:
R. Sander: Compilation of Henry's law constants (version 5.0.0) for
water as solvent, Atmos. Chem. Phys., 23, 10901-12440 (2023),
doi:10.5194/acp-23-10901-2023
The publication from 2023 replaces that from 2015,
which is now obsolete. Please do not cite the old paper anymore.
|
| FORMULA: | C2H5COOONO2 |
|
TRIVIAL NAME:
|
PPN
|
|
CAS RN: | 5796-89-4 |
STRUCTURE
(FROM
NIST):
|
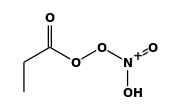
|
|
InChIKey: | TXINBPKSWKFMNB-UHFFFAOYSA-N |
|
|
References |
Type |
Notes |
| [mol/(m3Pa)] |
[K] |
|
|
|
| 1.6×10−2 |
6000 |
Easterbrook et al. (2023) |
M |
|
| 2.9×10−2 |
|
Kames and Schurath (1995) |
M |
12)
|
| 3.9 |
|
Wang et al. (2017) |
Q |
81)
239)
|
| 4.1 |
|
Wang et al. (2017) |
Q |
81)
240)
|
| 3.6×10−4 |
|
Wang et al. (2017) |
Q |
81)
241)
|
| 2.5×10−2 |
|
Raventos-Duran et al. (2010) |
Q |
243)
244)
|
| 2.0 |
|
Raventos-Duran et al. (2010) |
Q |
245)
|
| 6.2×10−2 |
|
Raventos-Duran et al. (2010) |
Q |
246)
|
|
|
Warneck et al. (1996) |
? |
585)
|
|
|
Schurath et al. (1996) |
W |
587)
|
Data
The first column contains Henry's law solubility constant
at the reference temperature of 298.15 K.
The second column contains the temperature dependence
, also at the
reference temperature.
References
-
Easterbrook, K. D., Vona, M. A., Nayebi-Astaneh, K., Miller, A. M., & Osthoff, H. D.: Measurement of Henry’s law and liquid-phase loss rate constants of peroxypropionic nitric anhydride (PPN) in deionized water and in n-octanol, Atmos. Chem. Phys., 23, 311–322, doi:10.5194/ACP-23-311-2023 (2023).
-
Kames, J. & Schurath, U.: Henry’s law and hydrolysis-rate constants for peroxyacyl nitrates (PANs) using a homogeneous gas-phase source, J. Atmos. Chem., 21, 151–164, doi:10.1007/BF00696578 (1995).
-
Raventos-Duran, T., Camredon, M., Valorso, R., Mouchel-Vallon, C., & Aumont, B.: Structure-activity relationships to estimate the effective Henry’s law constants of organics of atmospheric interest, Atmos. Chem. Phys., 10, 7643–7654, doi:10.5194/ACP-10-7643-2010 (2010).
-
Schurath, U., Bongartz, A., Kames, J., Wunderlich, C., & Carstens, T.: Chapter 6.4: Laboratory determination of physico-chemical rate parameters pertinent to mass transfer into cloud and fog droplets, in: Heterogeneous and Liquid-Phase Processes, edited by Warneck, P., pp. 182–189, Springer Verlag, Berlin, doi:10.1007/978-3-642-61445-3_6 (1996).
-
Wang, C., Yuan, T., Wood, S. A., Goss, K.-U., Li, J., Ying, Q., & Wania, F.: Uncertain Henry’s law constants compromise equilibrium partitioning calculations of atmospheric oxidation products, Atmos. Chem. Phys., 17, 7529–7540, doi:10.5194/ACP-17-7529-2017 (2017).
-
Warneck, P., Mirabel, P., Salmon, G. A., van Eldik, R., Vinckier, C., Wannowius, K. J., & Zetzsch, C.: Chapter 2: Review of the activities and achievements of the EUROTRAC subproject HALIPP, in: Heterogeneous and Liquid-Phase Processes, edited by Warneck, P., pp. 7–74, Springer Verlag, Berlin, doi:10.1007/978-3-642-61445-3_2 (1996).
Type
Table entries are sorted according to reliability of the data, listing
the most reliable type first: L) literature review, M) measured, V)
VP/AS = vapor pressure/aqueous solubility, R) recalculation, T)
thermodynamical calculation, X) original paper not available, C)
citation, Q) QSPR, E) estimate, ?) unknown, W) wrong. See Section 3.1
of Sander (2023) for further details.
Notes
| 12) |
Value at T = 293 K. |
| 81) |
Value at T = 288 K. |
| 239) |
Calculated using linear free energy relationships (LFERs). |
| 240) |
Calculated using SPARC Performs Automated Reasoning in Chemistry (SPARC). |
| 241) |
Calculated using COSMOtherm. |
| 243) |
Value from the training dataset. |
| 244) |
Calculated using the GROMHE model. |
| 245) |
Calculated using the SPARC approach. |
| 246) |
Calculated using the HENRYWIN method. |
| 585) |
Comparing the value with that from the cited publication (Kames and Schurath, 1995), it can be seen that the unit and the temperature listed in Table 3 of Warneck et al. (1996) are incorrect. |
| 587) |
The data from Kames and Schurath (1995) for peroxypropionyl nitrate are incorrectly cited by Schurath et al. (1996). |
The numbers of the notes are the same as
in Sander (2023). References cited in the notes can be
found here.
|
* * *
Search Henry's Law Database
* * *
Convert Henry's Law Constants
* * *
|

