When referring to the compilation of Henry's Law Constants, please cite
this publication:
R. Sander: Compilation of Henry's law constants (version 5.0.0) for
water as solvent, Atmos. Chem. Phys., 23, 10901-12440 (2023),
doi:10.5194/acp-23-10901-2023
The publication from 2023 replaces that from 2015,
which is now obsolete. Please do not cite the old paper anymore.
|
| FORMULA: | C8H14O |
|
TRIVIAL NAME:
|
trans-2-octenal
|
|
CAS RN: | 2548-87-0 |
STRUCTURE
(FROM
NIST):
|
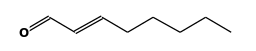
|
|
InChIKey: | LVBXEMGDVWVTGY-VOTSOKGWSA-N |
|
|
References |
Type |
Notes |
| [mol/(m3Pa)] |
[K] |
|
|
|
| 1.3×10−1 |
|
Buttery et al. (1971) |
M |
|
| 1.4×10−1 |
|
Yaffe et al. (2003) |
Q |
249)
250)
|
| 1.0×10−1 |
|
Nirmalakhandan et al. (1997) |
Q |
|
| 1.1×10−1 |
|
Suzuki et al. (1992) |
Q |
233)
|
|
|
Betterton (1992) |
W |
473)
|
Data
The first column contains Henry's law solubility constant
at the reference temperature of 298.15 K.
The second column contains the temperature dependence
, also at the
reference temperature.
References
-
Betterton, E. A.: Henry’s law constants of soluble and moderately soluble organic gases: Effects on aqueous phase chemistry, Adv. Environ. Sci. Technol., 24, 1–50 (1992).
-
Buttery, R. G., Bomben, J. L., Guadagni, D. G., & Ling, L. C.: Some considerations of volatilities of organic flavor compounds in foods, J. Agric. Food Chem., 19, 1045–1048, doi:10.1021/JF60178A004 (1971).
-
Nirmalakhandan, N., Brennan, R. A., & Speece, R. E.: Predicting Henry’s law constant and the effect of temperature on Henry’s law constant, Wat. Res., 31, 1471–1481, doi:10.1016/S0043-1354(96)00395-8 (1997).
-
Suzuki, T., Ohtaguchi, K., & Koide, K.: Application of principal components analysis to calculate Henry’s constant from molecular structure, Comput. Chem., 16, 41–52, doi:10.1016/0097-8485(92)85007-L (1992).
-
Yaffe, D., Cohen, Y., Espinosa, G., Arenas, A., & Giralt, F.: A fuzzy ARTMAP-based quantitative structure-property relationship (QSPR) for the Henry’s law constant of organic compounds, J. Chem. Inf. Comput. Sci., 43, 85–112, doi:10.1021/CI025561J (2003).
Type
Table entries are sorted according to reliability of the data, listing
the most reliable type first: L) literature review, M) measured, V)
VP/AS = vapor pressure/aqueous solubility, R) recalculation, T)
thermodynamical calculation, X) original paper not available, C)
citation, Q) QSPR, E) estimate, ?) unknown, W) wrong. See Section 3.1
of Sander (2023) for further details.
Notes
| 233) |
Calculated with a principal component analysis (PCA); see Suzuki et al. (1992) for details. |
| 249) |
Yaffe et al. (2003) present QSPR results calculated with the fuzzy ARTMAP (FAM) and with the back-propagation (BK-Pr) method. They conclude that FAM is better. Only the FAM results are shown here. |
| 250) |
Value from the training set. |
| 473) |
The data from Buttery et al. (1971) for trans-2-octenal are incorrectly cited by Betterton (1992). |
The numbers of the notes are the same as
in Sander (2023). References cited in the notes can be
found here.
|
* * *
Search Henry's Law Database
* * *
Convert Henry's Law Constants
* * *
|

