When referring to the compilation of Henry's Law Constants, please cite
this publication:
R. Sander: Compilation of Henry's law constants (version 5.0.0) for
water as solvent, Atmos. Chem. Phys., 23, 10901-12440 (2023),
doi:10.5194/acp-23-10901-2023
The publication from 2023 replaces that from 2015,
which is now obsolete. Please do not cite the old paper anymore.
|
| FORMULA: | C3H2ClF5O |
|
TRIVIAL NAME:
|
enflurane
|
|
CAS RN: | 13838-16-9 |
STRUCTURE
(FROM
NIST):
|
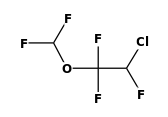
|
|
InChIKey: | JPGQOUSTVILISH-UHFFFAOYSA-N |
|
|
References |
Type |
Notes |
| [mol/(m3Pa)] |
[K] |
|
|
|
| 3.0×10−4 |
|
Fogg and Sangster (2003) |
L |
|
| 3.0×10−4 |
|
Allott et al. (1973) |
L |
14)
|
| 2.7×10−4 |
|
Guitart et al. (1989) |
M |
14)
|
| 2.9×10−4 |
|
Lerman et al. (1983) |
M |
14)
|
| 1.3×10−3 |
|
HSDB (2015) |
V |
|
| 3.0×10−4 |
|
Steward et al. (1973) |
C |
14)
|
| 6.9×10−4 |
|
Hilal et al. (2008) |
Q |
|
| 3.1×10−4 |
|
Abraham and Weathersby (1994) |
? |
21)
|
Data
The first column contains Henry's law solubility constant
at the reference temperature of 298.15 K.
The second column contains the temperature dependence
, also at the
reference temperature.
References
-
Abraham, M. H. & Weathersby, P. K.: Hydrogen bonding. 30. Solubility of gases and vapors in biological liquids and tissues, J. Pharm. Sci., 83, 1450–1456, doi:10.1002/JPS.2600831017 (1994).
-
Allott, P. R., Steward, A., Flook, V., & Mapleson, W. W.: Variation with temperature of the solubilities of inhaled anaesthestics in water, oil and biological media, Br. J. Anaesth., 45, 294–300, doi:10.1093/BJA/45.3.294 (1973).
-
Fogg, P. & Sangster, J.: Chemicals in the Atmosphere: Solubility, Sources and Reactivity, John Wiley & Sons, Inc., ISBN 978-0-471-98651-5 (2003).
-
Guitart, R., Puigdemont, F., & Arboix, M.: Rapid headspace gas chromatographic method for the determination of liquid/gas partition coefficients, J. Chromatogr., 491, 271–280, doi:10.1016/S0378-4347(00)82845-5 (1989).
-
Hilal, S. H., Ayyampalayam, S. N., & Carreira, L. A.: Air-liquid partition coefficient for a diverse set of organic compounds: Henry’s law constant in water and hexadecane, Environ. Sci. Technol., 42, 9231–9236, doi:10.1021/ES8005783 (2008).
-
HSDB: Hazardous Substances Data Bank, TOXicology data NETwork (TOXNET), National Library of Medicine (US), URL https://www.nlm.nih.gov/toxnet/Accessing_HSDB_Content_from_PubChem.html (2015).
-
Lerman, J., Willis, M. M., Gregory, G. A., & Eger, E. I.: Osmolarity determines the solubility of anesthetics in aqueous solutions at 37∘C, Anesthesiology, 59, 554–558, doi:10.1097/00000542-198312000-00013 (1983).
-
Steward, A., Allott, P. R., Cowles, A. L., & Mapleson, W. W.: Solubility coefficients for inhaled anaesthetics for water, oil and biological media, Br. J. Anaesth., 45, 282–293, doi:10.1093/BJA/45.3.282 (1973).
Type
Table entries are sorted according to reliability of the data, listing
the most reliable type first: L) literature review, M) measured, V)
VP/AS = vapor pressure/aqueous solubility, R) recalculation, T)
thermodynamical calculation, X) original paper not available, C)
citation, Q) QSPR, E) estimate, ?) unknown, W) wrong. See Section 3.1
of Sander (2023) for further details.
Notes
| 14) |
Value at T = 310 K. |
| 21) |
Several references are given in the list of Henry's law constants but not assigned to specific species. |
The numbers of the notes are the same as
in Sander (2023). References cited in the notes can be
found here.
|
* * *
Search Henry's Law Database
* * *
Convert Henry's Law Constants
* * *
|

