When referring to the compilation of Henry's Law Constants, please cite
this publication:
R. Sander: Compilation of Henry's law constants (version 5.0.0) for
water as solvent, Atmos. Chem. Phys., 23, 10901-12440 (2023),
doi:10.5194/acp-23-10901-2023
The publication from 2023 replaces that from 2015,
which is now obsolete. Please do not cite the old paper anymore.
|
| FORMULA: | NO |
|
TRIVIAL NAME:
|
nitric oxide
|
|
CAS RN: | 10102-43-9 |
STRUCTURE
(FROM
NIST):
|
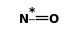
|
|
InChIKey: | MWUXSHHQAYIFBG-UHFFFAOYSA-N |
|
|
References |
Type |
Notes |
| [mol/(m3Pa)] |
[K] |
|
|
|
| 1.9×10−5 |
1600 |
Warneck and Williams (2012) |
L |
|
| 1.9×10−5 |
1600 |
Sander et al. (2011) |
L |
1)
77)
|
| 1.9×10−5 |
1600 |
Sander et al. (2006) |
L |
1)
78)
|
| 1.9×10−5 |
1500 |
Schwartz and White (1981) |
L |
|
| 1.9×10−5 |
1400 |
Young (1981b) |
L |
1)
79)
|
| 1.3×10−5 |
|
Zafiriou and McFarland (1980) |
M |
80)
|
| 2.3×10−5 |
|
Komiyama and Inoue (1980) |
M |
81)
|
| 1.9×10−5 |
1500 |
Komiyama and Inoue (1978) |
M |
|
| 1.9×10−5 |
1400 |
Winkler (1901) |
M |
82)
|
| 3.4×10−5 |
|
Pierotti (1965) |
T |
|
| 1.9×10−5 |
1300 |
Loomis (1928) |
C |
83)
|
| 1.5×10−5 |
|
Hayer et al. (2022) |
Q |
20)
|
|
1500 |
Kühne et al. (2005) |
Q |
|
|
1600 |
Kühne et al. (2005) |
? |
|
| 1.9×10−5 |
1400 |
Yaws et al. (1999) |
? |
21)
|
| 1.9×10−5 |
1500 |
Dean and Lange (1999) |
? |
23)
84)
|
| 1.9×10−5 |
|
Seinfeld (1986) |
? |
21)
|
| 1.9×10−5 |
|
Andrew and Hanson (1961) |
? |
|
|
|
Burkholder et al. (2019) |
W |
85)
|
|
|
Burkholder et al. (2015) |
W |
86)
|
|
|
Wilhelm et al. (1977) |
W |
87)
|
Data
The first column contains Henry's law solubility constant
at the reference temperature of 298.15 K.
The second column contains the temperature dependence
, also at the
reference temperature.
References
-
Andrew, S. P. S. & Hanson, D.: The dynamics of nitrous gas absorption, Chem. Eng. Sci., 14, 105–113, doi:10.1016/0009-2509(61)85060-4 (1961).
-
Burkholder, J. B., Sander, S. P., Abbatt, J., Barker, J. R., Huie, R. E., Kolb, C. E., Kurylo, M. J., Orkin, V. L., Wilmouth, D. M., & Wine, P. H.: Chemical Kinetics and Photochemical Data for Use in Atmospheric Studies, Evaluation No. 18, JPL Publication 15-10, Jet Propulsion Laboratory, Pasadena, URL https://jpldataeval.jpl.nasa.gov (2015).
-
Burkholder, J. B., Sander, S. P., Abbatt, J., Barker, J. R., Cappa, C., Crounse, J. D., Dibble, T. S., Huie, R. E., Kolb, C. E., Kurylo, M. J., Orkin, V. L., Percival, C. J., Wilmouth, D. M., & Wine, P. H.: Chemical Kinetics and Photochemical Data for Use in Atmospheric Studies, Evaluation No. 19, JPL Publication 19-5, Jet Propulsion Laboratory, Pasadena, URL https://jpldataeval.jpl.nasa.gov (2019).
-
Dean, J. A. & Lange, N. A.: Lange’s Handbook of Chemistry, Fifteenth Edition, McGraw-Hill, Inc., ISBN 9780070163843 (1999).
-
Hayer, N., Jirasek, F., & Hasse, H.: Prediction of Henry’s law constants by matrix completion, AIChE J., 68, e17 753, doi:10.1002/AIC.17753 (2022).
-
Komiyama, H. & Inoue, H.: Reaction and transport of nitrogen oxides in nitrous acid solutions, J. Chem. Eng. Jpn., 11, 25–32, doi:10.1252/JCEJ.11.25 (1978).
-
Komiyama, H. & Inoue, H.: Absorption of nitrogen oxides into water, Chem. Eng. Sci., 35, 154–161, doi:10.1016/0009-2509(80)80082-0 (1980).
-
Kühne, R., Ebert, R.-U., & Schüürmann, G.: Prediction of the temperature dependency of Henry’s law constant from chemical structure, Environ. Sci. Technol., 39, 6705–6711, doi:10.1021/ES050527H (2005).
-
Loomis, A. G.: Solubilities of gases in water, in: International Critical Tables of Numerical Data, Physics, Chemistry and Technology, Vol. III, edited by Washburn, E. W., West, C. J., Dorsey, N. E., Bichowsky, F. R., & Klemenc, A., pp. 255–261, McGraw-Hill, Inc. (1928).
-
Pierotti, R. A.: Aqueous solutions of nonpolar gases, J. Phys. Chem., 69, 281–288, doi:10.1021/J100885A043 (1965).
-
Sander, S. P., Friedl, R. R., Golden, D. M., Kurylo, M. J., Moortgat, G. K., Keller-Rudek, H., Wine, P. H., Ravishankara, A. R., Kolb, C. E., Molina, M. J., Finlayson-Pitts, B. J., Huie, R. E., & Orkin, V. L.: Chemical Kinetics and Photochemical Data for Use in Atmospheric Studies, Evaluation Number 15, JPL Publication 06-2, Jet Propulsion Laboratory, Pasadena, CA, URL https://jpldataeval.jpl.nasa.gov (2006).
-
Sander, S. P., Abbatt, J., Barker, J. R., Burkholder, J. B., Friedl, R. R., Golden, D. M., Huie, R. E., Kolb, C. E., Kurylo, M. J., Moortgat, G. K., Orkin, V. L., & Wine, P. H.: Chemical Kinetics and Photochemical Data for Use in Atmospheric Studies, Evaluation No. 17, JPL Publication 10-6, Jet Propulsion Laboratory, Pasadena, URL https://jpldataeval.jpl.nasa.gov (2011).
-
Schwartz, S. E. & White, W. H.: Solubility equilibria of the nitrogen oxides and oxyacids in dilute aqueous solution, in: Advances in Environmental Science and Engineering, edited by Pfafflin, J. R. & Ziegler, E. N., vol. 4, pp. 1–45, Gordon and Breach Science Publishers, NY (1981).
-
Seinfeld, J. H.: Atmospheric Chemistry and Physics of Air Pollution, Wiley-Interscience Publication, NY, ISBN 0471828572 (1986).
-
Warneck, P. & Williams, J.: The Atmospheric Chemist’s Companion: Numerical Data for Use in the Atmospheric Sciences, Springer Verlag, doi:10.1007/978-94-007-2275-0 (2012).
-
Wilhelm, E., Battino, R., & Wilcock, R. J.: Low-pressure solubility of gases in liquid water, Chem. Rev., 77, 219–262, doi:10.1021/CR60306A003 (1977).
-
Winkler, L. W.: Die Löslichkeit der Gase in Wasser (dritte Abhandlung), Ber. Dtsch. Chem. Ges., 34, 1408–1422, doi:10.1002/CBER.19010340210 (1901).
-
Yaws, C. L., Hopper, J. R., Wang, X., Rathinsamy, A. K., & Pike, R. W.: Calculating solubility & Henry’s law constants for gases in water, Chem. Eng., pp. 102–105 (1999).
-
Young, C. L.: IUPAC Solubility Data Series, Volume 8, Oxides of Nitrogen, Pergamon Press, Oxford, doi:10.1016/C2009-0-00222-0 (1981b).
-
Zafiriou, O. C. & McFarland, M.: Determination of trace levels of nitric oxide in aqueous solution, Anal. Chem., 52, 1662–1667, doi:10.1021/AC50061A029 (1980).
Type
Table entries are sorted according to reliability of the data, listing
the most reliable type first: L) literature review, M) measured, V)
VP/AS = vapor pressure/aqueous solubility, R) recalculation, T)
thermodynamical calculation, X) original paper not available, C)
citation, Q) QSPR, E) estimate, ?) unknown, W) wrong. See Section 3.1
of Sander (2023) for further details.
Notes
| 1) |
A detailed temperature dependence with more than one parameter is available in the original publication. Here, only the temperature dependence at 298.15 K according to the van 't Hoff equation is presented. |
| 20) |
Calculated using machine learning matrix completion methods (MCMs). |
| 21) |
Several references are given in the list of Henry's law constants but not assigned to specific species. |
| 23) |
The partial pressure of water vapor (needed to convert some Henry's law constants) was calculated using the formula given by Buck (1981). The quantities A and α from Dean and Lange (1999) were assumed to be identical. |
| 77) |
The H298 and A, B, C data listed in Table 5.4 of Sander et al. (2011) are inconsistent, with 94 % difference. |
| 78) |
The H298 and A, B, C data listed in Table 5.4 of Sander et al. (2006) are inconsistent, with 94 % difference. |
| 79) |
A minus sign is missing in the fitting parameter presented by Young (1981b). It should be −62.8086, not 62.8086. |
| 80) |
Value at T = 297 K. |
| 81) |
Value at T = 288 K. |
| 82) |
The data from Winkler (1901) were fitted to the three-parameter equation: Hscp= exp( −184.00012 +8924.34832/T +25.13228 ln(T)) mol m−3 Pa−1, with T in K. |
| 83) |
The data from Loomis (1928) were fitted to the three-parameter equation: Hscp= exp( −223.88313 +10620.37030/T +31.13453 ln(T)) mol m−3 Pa−1, with T in K. |
| 84) |
The data from Dean and Lange (1999) were fitted to the three-parameter equation: Hscp= exp( −160.19223 +7888.02642/T +21.56401 ln(T)) mol m−3 Pa−1, with T in K. |
| 85) |
Incorrect data are given by Burkholder et al. (2019) for NO. The correct parameters for the temperature dependence are A = −163.86, B = 8234, C = 22.816 (Robert E. Huie, personal communication, 2021). |
| 86) |
Incorrect data are given by Burkholder et al. (2015) for NO. The correct parameters for the temperature dependence are A = −163.86, B = 8234, C = 22.816 (Robert E. Huie, personal communication, 2021). |
| 87) |
The fitting parameters A, B, C, and D in Table I of Wilhelm et al. (1977) do not reproduce the data in their Table III. |
The numbers of the notes are the same as
in Sander (2023). References cited in the notes can be
found here.
|
* * *
Search Henry's Law Database
* * *
Convert Henry's Law Constants
* * *
|

